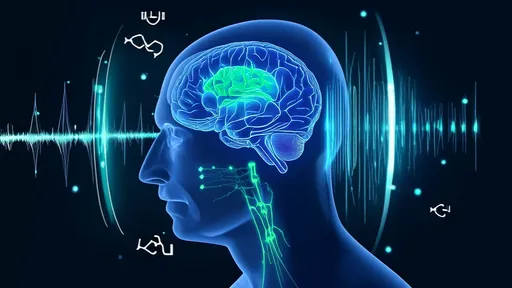
In a groundbreaking leap for neuroscience and bioengineering, researchers have unveiled a revolutionary technique that merges ultrasound technology with genetic engineering to noninvasively control deep brain activity. This innovation, termed ultrasonic neuro-modulation, promises to transform how we study and treat neurological disorders by acting as a genetic switch for targeted brain regions—without the need for invasive surgery.
The approach builds on the growing field of sonogenetics, which uses sound waves to manipulate genetically modified neurons. Unlike traditional deep brain stimulation (DBS), which requires implanted electrodes, this method relies on focused ultrasound waves to activate or silence specific neural circuits. By combining ultrasound with engineered ion channels sensitive to sound pressure, scientists can now reach areas like the hippocampus or thalamus with unprecedented precision, bypassing the blood-brain barrier and minimizing collateral damage.
Early experiments in animal models have demonstrated the technique’s potential. In one study, researchers genetically modified neurons in mice to express mechanosensitive ion channels, which respond to ultrasonic waves. When exposed to focused ultrasound, these channels opened, triggering neuronal firing. Conversely, inhibitory channels could suppress activity, effectively creating an on-off switch for brain circuits. This level of control could pave the way for treating conditions like Parkinson’s disease, epilepsy, or even psychiatric disorders where dysregulated brain regions are deeply embedded.
What sets this apart from other noninvasive methods, such as transcranial magnetic stimulation (TMS), is its spatial resolution and depth penetration. While TMS struggles to target subcortical structures without affecting overlying areas, ultrasound can be finely tuned to focus energy millimeters-wide, deep within the brain. Moreover, the genetic component allows for cell-type specificity—targeting only neurons engineered to respond to sound, leaving surrounding tissue unaffected.
Ethical and safety considerations remain at the forefront. Unlike optogenetics, which requires invasive fiber optics, sonogenetics is minimally intrusive. However, long-term effects of chronic ultrasound exposure on brain tissue are still under investigation. Researchers emphasize that human applications will require rigorous testing, particularly to ensure that genetic modifications are reversible or confined to therapeutic contexts.
The implications extend beyond medicine. Neuroscientists envision using this tool to map intricate brain networks or study how specific neuron populations contribute to behavior. For instance, selectively silencing amygdala neurons could elucidate their role in fear responses, while activating dopaminergic circuits might reveal new insights into addiction pathways. The ability to toggle neural activity with a handheld ultrasound device—no surgery needed—could democratize brain research, making advanced manipulation techniques accessible to more labs.
Industry partnerships are already emerging to translate this technology into clinical tools. Startups are developing portable ultrasound emitters paired with viral vectors for safe gene delivery. Meanwhile, regulatory agencies are grappling with frameworks to oversee these hybrid therapies. The U.S. FDA recently fast-tracked a related approach using ultrasound to open the blood-brain barrier for drug delivery, signaling growing acceptance of acoustic technologies in neurology.
Critics caution that challenges persist. Delivering genetic constructs to human brains remains complex, and immune responses to viral vectors—a common gene therapy tool—must be carefully managed. Yet, proponents argue that the payoff justifies the hurdles. Imagine treating depression by calibrating activity in the subgenual cingulate cortex, or enhancing memory by stimulating hippocampal circuits—all through outpatient procedures.
As the field accelerates, interdisciplinary collaboration will be key. Bioengineers are refining ultrasound transducers for deeper penetration, while molecular biologists design safer, more responsive ion channels. Clinicians, meanwhile, are identifying patient populations who could benefit most. This convergence of expertise underscores a larger trend in modern medicine: the fusion of tools from physics, genetics, and neuroscience to rewrite therapeutic playbooks.
For now, ultrasonic neuro-modulation stands as a testament to human ingenuity—a literal remote control for the brain that could one day turn science fiction into standard care. As one lead researcher remarked, We’re not just knocking on the brain’s door anymore. We’ve found a way to walk through it—without breaking anything. The next decade may well reveal whether this technique can unlock the brain’s deepest secrets, one sound wave at a time.
By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025