The vagus nerve, a critical component of the parasympathetic nervous system, has emerged as a fascinating target for therapeutic interventions aimed at modulating inflammatory responses. Recent advances in neuroimmunology have unveiled the intricate pathways through which the vagus nerve communicates with the immune system, forming the basis of what is now known as the "inflammatory reflex." This reflex represents a neural circuit that detects and regulates inflammation, offering novel opportunities for treating chronic inflammatory diseases.
The inflammatory reflex operates through a feedback loop involving the vagus nerve, which senses peripheral inflammation and relays signals to the brainstem. In turn, efferent vagal signals are sent back to the spleen and other lymphoid organs, releasing neurotransmitters like acetylcholine that suppress pro-inflammatory cytokine production. This cholinergic anti-inflammatory pathway has been extensively studied in conditions such as rheumatoid arthritis, inflammatory bowel disease, and sepsis, where dysregulated inflammation plays a central role.
Targeting the vagus nerve to modulate inflammation is not a new concept, but recent technological advancements have revitalized interest in this approach. Bioelectronic medicine, for instance, explores the use of electrical stimulation to activate or inhibit specific neural pathways. Vagus nerve stimulation (VNS), both invasive and non-invasive, has shown promise in clinical trials for reducing inflammation in autoimmune diseases. The ability to precisely control neural activity opens doors to personalized therapies with fewer side effects compared to traditional immunosuppressive drugs.
One of the most compelling aspects of vagus nerve interventions is their potential to address the root cause of inflammation rather than merely alleviating symptoms. Chronic inflammation is often driven by a failure of natural regulatory mechanisms, and by enhancing the body's own anti-inflammatory pathways, VNS offers a more holistic approach. Studies have demonstrated that VNS can reduce levels of tumor necrosis factor-alpha (TNF-α) and other inflammatory markers, providing relief for patients with treatment-resistant conditions.
Despite these promising developments, challenges remain in optimizing vagus nerve-based therapies. The anatomy of the vagus nerve is complex, with branches that innervate multiple organs, making it difficult to achieve selective modulation without off-target effects. Researchers are now mapping the vagus nerve with unprecedented precision, using techniques like single-cell RNA sequencing and advanced imaging to identify specific neuronal populations involved in the inflammatory reflex. This "vagus nerve atlas" could pave the way for more targeted interventions.
Another area of active investigation is the development of biomarkers to predict patient responses to VNS. Not all individuals with inflammatory diseases may benefit equally from vagus nerve stimulation, and identifying those most likely to respond is crucial for clinical success. Combining neural stimulation with immune profiling may help tailor therapies to individual patients, maximizing efficacy while minimizing unnecessary procedures.
The intersection of neuroscience and immunology has also sparked interest in non-pharmacological approaches to inflammation management. Lifestyle factors such as meditation, deep breathing, and yoga, which enhance vagal tone, are being explored as adjuncts to conventional treatments. These practices may amplify the body's natural anti-inflammatory responses, offering a low-cost and accessible complement to bioelectronic therapies.
Looking ahead, the field of vagus nerve-targeted interventions is poised for significant growth. Ongoing research aims to refine stimulation protocols, improve device miniaturization, and expand the range of treatable conditions. Beyond inflammation, the vagus nerve's influence on metabolism, mood, and gut-brain communication suggests broader applications for neuro-modulatory therapies. As our understanding of the inflammatory reflex deepens, so too does the potential to harness the vagus nerve for healing.
In conclusion, the vagus nerve stands at the frontier of a new era in medicine, where neural circuits are manipulated to restore immune balance. While much work remains to translate laboratory findings into widespread clinical practice, the progress thus far underscores the transformative potential of targeting the inflammatory reflex. For patients with chronic inflammatory diseases, these advances offer hope for more effective and sustainable treatments in the years to come.
By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025

By /Aug 5, 2025
By /Aug 5, 2025
By /Aug 5, 2025
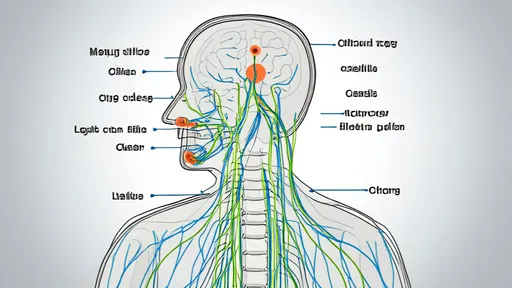
By /Aug 5, 2025